Sphincter Mechanism
Role of the Sphincter in Continence
A sphincter is traditionally defined as an annular, or circular, muscle designed to occlude an opening of the body.
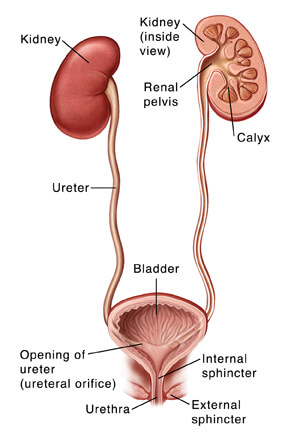
Two urethral sphincters (Figure X1) have been described as acting together and in concert with the periurethral muscles of the levator ani to maintain closure of the urethra during bladder filling and storage:
- an internal sphincter, composed of the smooth muscle of the bladder neck, and
- an external sphincter, composed of the intrinsic striated muscle fibers of the urethra.
Interestingly, advances in our understanding of lower urinary tract physiology have led to a more accurate and complete description of the urethral sphincter mechanism as a mixture of compressive, tension, and supportive elements.
During bladder filling and storage, these elements form a watertight urethral seal to prevent urinary leakage, even in the presence of sudden or strenuous physical exertion or precipitous rises in abdominal and intravesical pressure caused by coughing or sneezing.
In addition, this remarkable mechanism accommodates bladder evacuation by providing a low-resistance conduit for urinary outflow during micturition.
Elements of Compression
The compressive elements of the urethral sphincter mechanism are necessary to provide a watertight seal against the passage of urine from the bladder into the urethra during bladder filling and storage. The primary compressive elements include inner urethral softness, the mucoid lining of the urethral epithelium, and the submucosal vascular cushion.
- Urethral Softness
Inner urethral softness is the first essential compressive component of the urethral sphincter. In the physiologically normal individual, the urethral epithelium rapidly alters its shape to maintain a watertight seal during storage and to allow an unobstructed flow of urine during micturition.
This ability to deform itself to create a watertight seal can be observed when one is passing a stiff catheter into the bladder. Even though the catheter is smaller than the potential diameter of the urethra, urine exits exclusively through the lumen of the catheter rather than around the tube. This occurs because the inner lining of the urethra deforms to the shape of the catheter and forms a watertight seal around the catheter.
Unfortunately, prolonged catheterization may cause erosion of the urethral lining; in this case, leakage of urine around the tube occurs passively or with minimal exertion, even if a relatively large-diameter (French size) catheter is placed. Leakage around an indwelling catheter may also occur as a result of bladder spasms, which act to force urine out around the catheter; this phenomenon can occur whenever the bladder is abnormally irritable, as in the setting of bladder infection, constipation Opens in new window, or use of an overly large catheter or balloon.
- Surface tension
The second element of compression is the mucoid-like GAG layer within the urethra. GAG molecules alter the surface tension of the urethral epithelium, thus promoting coaptation and closure of the urethral walls. The combined effects of urethral softness and GAG molecules within the urethral wall can be conceptualized by the example of a soft condom. If a condom is unrolled and held by its distal end, the walls of this tubular structure will fall together, reflecting their softness.
If the condom is shaken briskly, its walls easily fall apart, indicating low surface tension (that is, a low propensity of the walls to cling together). However, if the walls of the condom are coated with a water-soluble lubricant (such as K-Y Jelly), they will tend to “coapt,” or adhere to one another, even if the condom is again shaken briskly; this is the result of the higher surface tension produced by the addition of the lubricant. The GAG lining of the urethral epithelium increases surface tension in a comparable manner.
- Vascular Cushion
The vascular cushion located immediately beneath the urethral epithelium is the third element contributing to urethral compression. This rich network of arteries, veins, and arteriovenous communications contributes to the compressive effects of the muscular elements of the sphincter; that is, the cushion acts as an incompressible sponge, thus transmitting the compressive forces to the urethral epithelium.
The volume of blood contained within the vascular cushion of the urethra is evidenced by the surprisingly high volume of blood loss that occurs after transurethral surgery or traumatic catheterization.
The effect of deficiencies in the compressive elements of the urethra can be illustrated by the occurrence of stress urinary incontinence and obstructed voiding in a male patient after radical prostatectomy. In this case, the softness of the urethral epithelium has been disrupted by scarring at the anastomotic site.
As a result, the urethral walls are unable to coapt (close) effectively, and this results in stress pattern incontinence; in addition, the loss of urethral softness prevents “funneling” of the urethra and therefore causes obstructed voiding (bladder outlet obstruction).
In women, estrogen is known to promote cellular reproduction in the urethral epithelium, to stimulate mucus production in both the vagina and the urethra, and to sustain the submucosal vascular cushion. These estrogenic effects may partly explain the association between estrogen deficiency and stress pattern incontinence and the complementary role of estrogens in the management of stress incontinence in women.
Elements of Tension
The smooth and striated muscles of the proximal portion of the urethra in the male, the muscular elements of the entire female urethra, and the periurethral striated muscle comprise the tension elements of the urethral sphincter mechanism (Figure X2).
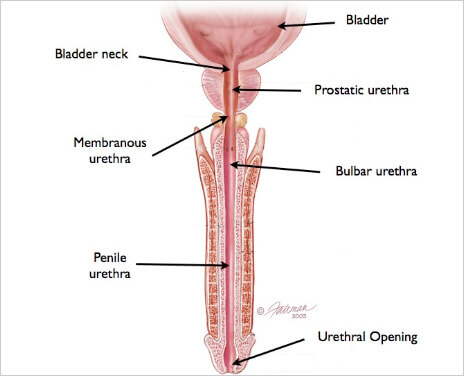
|
| Figure X2 | Muscular components of the male urethral sphincter mechanism |
- Smooth Muscle
Circular bundles of smooth muscle are found at the bladder neck of males; their importance to continence remains somewhat controversial. Contraction of the smooth muscle of the bladder neck is known to support antegrade ejaculation of semen, and surgical preservation of the bladder neck during radical prostatectomy has been found to promote early recovery of continence.
In addition, dyssynergic contraction of the smooth muscle of the bladder neck during voiding causes significant obstruction. All these findings support a significant role for this component of urethral smooth muscle, but the exact importance remains unclear.
Longitudinal smooth muscle bundles are also found within the urethral wall; contraction of these bundles may shorten and widen (funnel) the urethra during micturition or may promote urethral closure during bladder filling and storage.
Of important note, smooth muscle bundles are also found within the prostate gland, and their tone affects the prostatic urethra. An increase in resting tone is seen with aging and benign prostatic smooth muscle interferes with funneling of the prostatic urethra and may contribute to the obstruction associated with BPH.
In the female, smooth muscle bundles extend nearly the entire length of the urethra, but they thin significantly as they approach the meatus. The easily identifiable circular layer of smooth muscle present within the bladder neck of the female. Instead, smooth muscle bundles run obliquely or longitudinally along almost the entire urethral course.
Similar to the controversy noted in males, some investigators hypothesize that smooth muscle bundles contract during micturition to shorten and widen the urethra, whereas other investigators postulate that these bundles contribute to urethral narrowing and sphincter closure during bladder filling and storage.
- Rhabdosphincter
The intrinsic striated muscle of the urethral sphincter is called the rhabdosphincter. It is located in the membranous urethra, just below the apex of the prostate gland in the male and the middle third of the urethra in the female. In both genders it consists of unique omega-shaped fibers, and it thins as it joins connective tissue in the posterior urethra. Most of these fibers (65% to 100%) are Type 1, or slow-twitch fibers, which are ideally suited to maintaining tone over prolonged periods of time between voiding.
The rhabdosphincter is thought to be the most important element of tension in the competent urethral sphincter mechanism.
- Periurethral Striated Muscle
The periurethral striated sphincter of the female is divided into three distinctive portions:
- the sphincter urethrae,
- compressor urethrae, and
- urethral vaginal sphincter (see Figure X3).
These muscles contain both fast-twitch and slow-twitch striated muscle fibers that increase urethral closure pressure during brief periods of physical exertion or in response to voluntary commands.
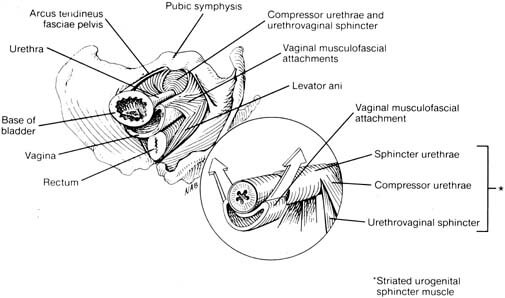
|
| Figure X3 | The female urethra and muscular components of the female rhabdosphincter: sphincter urethrae, urethrovaginal sphincter, and compressor urethrae. |
In the male, the periurethral striated muscle is located adjacent to the membranous urethra. Similar to the female, it contains a mixture of slow-twitch and fast-twitch skeletal muscle fibers, providing for rapid contraction in response to sudden rises in abdominal pressure and for maintenance of tone for prolonged periods, which is needed to ensure continence during routine physical activity.
The rhabdosphincter, periurethral muscles, and pelvic floor muscles also contribute to the individual’s ability to delay voiding; this is because sustained contraction of the pelvic muscled stimulates a spinal reflex that inhibits detrusor contraction. This has clinical significance, in that patients with urge pattern incontinence can be taught (usually with biofeedback) to voluntarily contract the pelvic muscles in order to inhibit bladder contraction and delay voiding.
Elements of Support
The pelvic floor provides support for the bladder and urethra, which is essential to competence of the urethral sphincter. The pelvic floor Opens in new window contributes to continence by maintaining the bladder base in an intraabdominal position. This position promotes transmission of abdominal pressure to both the bladder and the urethra, which is thought to assist with maintenance of urethral closure during activities that cause a sudden increase in abdominal pressure (such as coughing or sneezing).
The primary support structure is the levator ani muscle. The levator ani contains an abundance of slow-twitch muscle fibers, which maintain tone throughout the hours that a person remains in the upright position; it also contains fast-twitch fibers that are able to respond rapidly to precipitous rises in abdominal pressure. The endopelvic fascia provides supplemental support. It is important to remember that these structures are designed to provide dynamic support for the pelvic viscera. This flexibility is necessary for the passage of urine and stool and for the extraordinary demands created by vaginal delivery.
Several characteristics of the levator ani contribute to the confusion and contradictions that pervade our understanding of this remarkable muscle.
Unlike the muscles of the arms, legs, or chest, the levator ani is rarely viewed in its entirety, and hypertrophy of this muscle is not readily apparent to the unaided eye. As a result, it is often difficult for men and women to distinguish contraction of the levator ani muscle from contraction of the abdominal, thigh, or gluteal muscles.
In addition, exhaustion of the levator ani is not easily recognized, because repeated, purposive contraction does not produce the panting or muscle aches typically associated with muscle fatigue. This may result in subconscious substitution of the abdominal or gluteal muscles when the levator becomes fatigued.
Finally, the levator muscle, unlike the large muscles of the extremities, is primarily composed of slow-twitch fibers, and our understanding of effective techniques for exercising these muscle fibers is limited. Because of the unique characteristics of this muscle, effective rehabilitation typically biofeedback and repeated monitoring by a qualified health care professional.
Neurologic Modulation of the Sphincter Mechanism
The smooth muscle of the urethral sphincter mechanism contains both cholinergic and adrenergic receptors; adrenergic receptors promote urethral closure during bladder filling and storage, and cholinergic receptors promote urethral relaxation and funneling during micturition (see Table X1).
| Table X1 | Principal Receptors within the Lower Urinary Tract and their Pharmacologic Significance | ||
|---|---|---|
| Neurotransmitter/Physiologic Action | Associated Receptors | Pharmacologic Significance |
| Acetylcholine Stimulates contraction of smooth and striated muscle | Smooth muscle Muscarinic Muscarinic subtypes: M1 to M Striated muscle nicotinic | Cholinergic agents used to stimulate bladder muscle contraction (bethanechol chloride). Antimuscarinic agents: inhibit overactive detrusor contractions Oxybutynin (nonspecific, readily penetrates CNS) Tolterodine (evidence of organ but not receptor specificity, less likely to penetrate CNS) Trospium (poor penetrance into CNS) Solefenacin (receptor specificity, higher affinity for M3 receptors) Darifenacin (receptor specificity, higher affinity for M3 receptors) |
| Norepinephrine Stimulates smooth muscle contraction (via alpha- adrenergic receptors) or smooth muscle relaxation (via beta-2 or beta-3 receptors) | Smooth muscle Beta-3 adrenergic receptors in the bladder body Beta-2 adrenergic receptors in bladder neck Alpha-1a adrenergic receptors in urethral (and prostatic) smooth muscle | Nonspecific beta-adrenergic agents not found effective in the treatment of overactive bladder dysfunction (primarily because of intolerable cardiovascular or pulmonary side effects) but beta-3 specific agonists currently under investigation may overcome these limitations. Nonspecific alpha-adrenergic agonists increase bladder outlet resistance Ephedrine Pseudoephedrine Imipramine Alpha-adrenergic antagonists reduce bladder outlet resistance in bladder neck and urethra resulting from benign prostatic hypertrophy Terazosin (long-acting alpha-1 antagonist) Doxazosin (long-acting alpha-1 antagonist) Tamsulosin (greater receptor specificity to alpha-1a adrenergic subtypes reduces associated hypotension) Alfuzosin (greater receptor specificity to alpha-1a adrenergic subtypes reduces associated hypotension, reduced risk for retrograde ejaculation when compared with tamsulosin, doxazosin, or terazosin) |
During voiding, cholinergic (muscarinic) receptors within the urethra are thought to mediate contraction that reduces urethral length and increases urethral diameter; muscarinic receptors may also indirectly facilitate micturition by inhibiting norepinephrine release. During storage, adrenergic receptors mediate increased urethral smooth muscle tone, which is responsible for as much as 50% of urethral closure pressure.
Specifically, urethral closure is promoted by the action of the neurotransmitter norepinephrine which acts on alpha-1a adrenergic receptors, found throughout the urethral (and prostatic) smooth muscle.
Knowledge of specific receptor subtypes is clinically relevant because this knowledge has led to the development of pharmacologic agents that selectively antagonize alpha-1a receptors; these agents produce urethral sphincter relaxation in men with obstruction resulting from BPH without significantly lowering blood pressure. (The risk for hypotension or dizziness in men being managed by most alpha-adrenergic blocking agents is significant, because both the prostatic and arteriolar smooth muscles contain rich supplies of alpha-1 receptors; however, those in the prostate are predominantly alpha-1a subtypes, whereas those in the anterior smooth muscle are predominantly alpha-1b receptor subtypes.)
The bladder neck and urethral receptors, but this knowledge has not yet led to any effective pharmacologic agents for the management of stress urinary incontinenc or BPH, probably because beta-2 receptors are also abundant in the lungs.
Cholinergic receptors are also abundant in both the periurethral and pelvic floor striated muscles. However, the receptors for these striated muscles are primarily nicotinic rather than muscarinic, and they respond to different pharmacologic agonists or antagonists than does the smooth muscle of the lower urinary tract.
The type and physiologic role of receptors within the rhabdosphincter remain controversial. As is true of the pelvic floor and periurethral striated muscles, nicotinic receptors predominate within the striated fibers of the rhabdosphincter; however, there is some evidence that the rhabdosphincter may also be innervated by autonomic fibers, specifically by adrenergic nerve fibers.
It is particularly important to understand central nervous system modulation of the smooth and striated muscle of the sphincter mechanism and pelvic floor muscles because this is the basis for pharmacologic intervention in the management of stress urinary incontinence.
The smooth muscle of the urethra is innervated by branches of the autonomic nervous system; parasympathetic (cholinergic) nerves synapse with neurons at sacral segments 2 to 4, whereas adrenergic (sympathetic) nerves synapse with neurons in the spinal cord segments T10 to L2.
Innervation of the striated muscle of the urethra arises from branches of the pudendal (and not the pelvic) nerve, which synapse with neurons at Onuf’s nucleus (a collection of motor neurons located at the lateral border of the ventral horn of sacral segments 2 to 4).
During bladder filling and storage, activation of afferent pathways in the bladder provokes sphincter closure, primarily under the influence of reflex mechanisms within the spinal cord.
Specifically, neurons within Onuf’s nucleus mediate contraction of the rhabdosphincter via the neurotransmitter glutamine. This neurotransmitter may be thought of as activating an “on/off” mechanism that promotes sphincter closure during bladder filling (the “on” position) and inhibits contraction to allow urethral opening for micturition (the “off” switch).
As noted previously, these actions are coordinated by the pontine micturition center. As long as glutamine is physiologically active, the tone of the rhabdosphincter can be further modulated by two additional neurotransmitters produced by neurons in Onuf’s nucleus: norepinephrine and serotonin.
However, when glutamine is transiently eliminated in preparation for micturition, the activity of these complementary neurotransmitters is also eliminated, thus ensuring that micturition occurs without resistance from a partially contracted rhaddosphincter.
The clinical significane of these pathways and neurotransmitters is reflected in recent development of an investigation drug for the treatment of stress incontinence. This agent, duloxetine, acts on neurons within the spinal cord to increase urethral resistance by increasing the availability of the neurotransmitters serotonin and norepinephrine. These neurotransmitters help to modulate striated muscle tone during bladder filling, as explained above.
See also:
- Moore KN, Paul P: A historical review of selected nursing and medical literature on urinary incontinence between 1850 and 1976, J Wound Ostomy Continence Nurs 24:106-122, 1997.
- Cooper CS, Abousally CT, Austin JC, et al: Do public schools teach voiding dysfunction: Results of an elementary school teacher survey, J Urol 170(3):956-958, 2003.
- Fitzgerald ST, Palmer MH, Kirkland VL, Robinson L: The impact of urinary incontinence in working women: a study in a production facility, Womens Health 35(1):1-16, 2002.
- Steers WD: Physiology of the urinary bladder. In Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors: Campbell’s urology, ed 6, Philadelphia, 1992, WB Saunders.
- Steers WD: Physiology and pharmacology of the bladder and urethra. In Walsh PC, Retik AB, Vaughan ED, Wein EJ, editors: Campbell’s urology, ed 7, Philadelphia, 1998, WB Saunders.
- Gray ML: Geniourinary disorders, St. Louis, 1992, Mosby.
- Dixon J, Gosling J: Structure and innervations in the human. In Torrens M, Morrison JFB, editors: Physiology of the lower urinary tract, London, 1987, Springer-Verlag.
- Elbadawi A: Anatomy and innervations of the vesicourethral muscular unit of micturition. In Krane RJ, Siroky MB, editors: Clinical neuro-urology, Boston, 1991, Little, Brown.
- Rosier PFWM: Bladder function in elderly male patients. Thesis presented to the Department of Urology, School of Medicine, University of Nijmegen, The Netherlands, 1996.
- de Groat WC, Fraser MO, Yoshiyama M, et al: Neural control of the urethra, Scand J Urol Nephrol Suppl 207:35-43, 2001.
- Zderic SA, Chacko S, DiSanto ME, Wein AJ: Voiding function: relevant anatomy, physiology, pharmacology and molecular aspects. In: Gillenwater JY, Grayhack JT, Howards SS, Mitchell ME, editors: Adult and pediatric urology, ed 4, Philadelphia, 2002, Williams & Wilkins, pp 1061-1113.
- Weiss RM: Physiology and pharmacology of the renal pelvis and ureter. In Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors: Campbell’s urology, ed 7, Philadelphia, 1998, WB Saunders.
- Baker JC, Mitteness LS: Nocturia in the elderly, Gerontologist 28:99-104, 1988.
- Brading AF, Mostwin JL: Electrical and mechanical responses of guinea-pig bladder muscle to nerve stimulation, Br J Pharmacol 98 (4):1083-1090, 1989.
- Parekh AB, Brading AF, Tomita T: Studies of longitudinal tissue impedance in various smooth muscles, Prog Clin Biol Res 327:375-378, 1990.
- DeLancey JO: Functional anatomy of the female pelvis. In Kursh ED, McGuire EJ, editors: Female urology, Philadelphia, 1994, JB Lippincott.
- Brooks JD: Anatomy of the lower urinary tract and male genitalia. In Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors: Campbell’s urology, ed 7, Philadelphia, 1998, WB Saunders.
- Wahle GR, Young GPH, Raz S: Anatomy and physiology of pelvic support. In Raz S, editor: Female urology, Philadelphia, 1996, WB Saunders.
- Redman JF: Anatomy of the genitourinary system. In Gillenwater JY, Grayhack JT, Howards SS, Dukett JW, editors: Adult and pediatric urology, St. Louis, 1996, Mosby.
- Siroky MB: Electromyography of the perineal striated muscles. In Krane RJ, Siroky MB, editors: Clinical neurology, Boston, 1991, Little, Brown.
- Couillard DR, Webster GD: Detrusor instability, Urol Clin North Am 22(3):593-612, 1995.
- Wein AJ, Rovner ES. Definition and epidemiology of overactive bladder, Urology 60(5 suppl 1):7-12, 2002.
- Abrams RM, Stanley H, Carter R, Notelovitz M: Effect of conjugated estrogen on vaginal blood flow in surgically menopausal women, Am J Obstet Gynecol 143:375, 1985.
- Gray M: Urodynamic evaluaton of detrusor instability. Doctoral dissertation, University of Florida, Gainesville, Fla, 1990.
- Zinner NR: Clinical aspects of detrusor instability and the value of urodynamics, Eur Urol 34(suppl 1): 16-19, 1998.
- Gillespie JI, Harvey IJ, Drake MJ: Agonist- and nerve-induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity, Exp Physiol 88(3):343-357, 2003.
- Klausner AP, Steers WD: Research frontiers in the treatment of urinary incontinence, Clin Obstet Gynecol 47(1):104-113, 2004.
- Andrew J, Nathan PW, Spanos NC: Cerebral cortex and micturition, Proc R Soc Med 58:533, 1964.
- Athwal BS, Berkley KJ, Hussain I, et al: Brain responses to changes in bladder volume and urge to void in healthy men, Brain 124(2):369-377, 2001.
- Blok BF, Sturms LM, Holstage G: Brain activation during micturition in women, Brain 121(11):2033-2042, 1998.
- Matsuura S, Kakizaki H, Mitsui T, et al: Human brain region response to distention or cold stimulation of the bladder: a position emission tomography study, J Urol 168(5):2035-2039, 2002.
- Nour S, Svarer C, Kristensen JK, et al: Cerebral activation during micturition in normal men, Brain 123(4):781-789, 2000.
